-
-
- On this page : Lithium &
Ceramics, by Smart2000 (translated by Edouard
Bastarache)
-
-
|
LITHIUM
& CERAMICS
by
Smart2000
|
-
-
- This alkaline metal
is the lightest of known metals (Li, atomic mass
6.941).
-
-
- In ceramics it is found
in the oxidized state, Li2O (molar mass
29.881
-
-
- Classified as the 33th most
abundant element on the planet, this metal is present
in deposits coming from salt lakes, deposits of
silicates (spodumene, petalite, lepidolite), of
phosphates (amblygonite), of phyllosilicates coming
from the modification of volcanic ash (ex: hectorite
in California) and of borates like jadarite
(Serbia).
-
- Today the demand for this
metal for the production of lithium-ion batteries used
in data processing and telephony is enormous. Even if
it is used rather little outside of ceramics its price
currently beats records.
-
- Lithium oxide, Li2O, is the
most potent flux among alkaline oxides, one can
classify it as follows: Li2O > Na2O > K2O. It is
a very active flux which strongly decreases the
viscosity of glazes. According to the proportions
used, it lowers also the thermal dilatation
coefficient of glazes and of ceramic bodies for high
temperature.
-
-
- In current ceramics
lithium is useful :
-
- - In flameware bodies
intended to produce cooking pieces going directly on
the flame (see also the article " Flameware
" by Ron Probst on Smart.Conseil). Li2O reduces the
thermal expansion and improves the properties of
resistance to thermal shock.
-
- - In glazes. Li2O,
introduced from chemicals (carbonate of lithium,
lithium fluoride) or natural mineral compounds
(spodumene, petalite,…), is a powerful flux in
particular when associated with potassium and sodium
feldspars.
- Its melting action begins at
750-780°C. It allows to strongly lower the
viscosity of molten glazes. Used up to 1 to 2% it can
reduce the maturation temperature of a glaze by 50 to
100°C.
- In important additions (>
3%) it strongly reduces the thermal dilatation
coefficient and can lead to shivering (shivering is
the opposite of crazing). Lithium also improves the
resistance to scratching.
-
-
- As a general rule one uses
lithium carbonate when one wants to introduce lithium
alone into a glaze. The melting point of lithium
carbonate is 723°C (in air).
- Lithium carbonate is not
very soluble (13.1 g. per liter of water at 20°C)
contrary to other salts of alkaline elements. This
solubility decreases with the rise in
temperature.
-
-
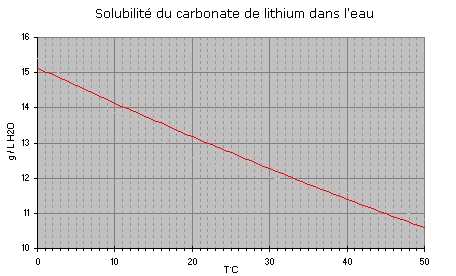
- Solubility of
lithium carbonate in water
-
- The least soluble raw
materials containing lithium are: lithium carbonate,
lithium frits (rather rare alas…) and minerals
such as spodumene, lithium feldspar, amblygonite and
lepidolite.
-
- In high temperature glazes,
Li2O attacks silica and alumina of the shard, which
allows the formation of an effective transition layer
to lower the tensions of dilatation. That also makes
it possible to make at equal temperature glazes with
more silica and alumina, more stable and more
resistant.
-
-
-
-
- Molar factors of alkaline
oxides for the calculation of the elasticity of
multi-component glasses according to A.A Appen :
-
-
- Li2O : 78.45 Gpa (ou 8 x
10-5 kgf/cm²)
- Na2O : 58.35 GPa (ou 5.95 x
10-5 kgf/cm²)
- K2O : 40.21 GPa (ou 4.1 x
10-5 kgf/cm²)
-
- Li2O increases the
elasticity modules of glazes (approximately twice as
much as K2O).
-
- Chart of natural and
chemical compounds :
-
-
-
|
Mineral Compounds
:
|
Chemical
Formula
|
Li2O%
|
Al2O3%
|
SiO2%
|
Molar
Mass
|
|
Aluminosilicates
:
|
-
|
-
|
-
|
-
|
-
|
|
Eucryptite
(E)*
|
Li2O.Al2O3.2SiO2
|
11.86
|
40.46
|
47.68
|
252
g
|
|
Spodumene
(S)*
|
Li2O.Al2O3.4SiO2
|
8.03
|
27.40
|
64.57
|
372.16
g
|
|
Lithium Feldspar
(R)*
|
Li2O.Al2O3.6SiO2
|
6.07
|
20.71
|
73.22
|
492.32
g
|
|
Petalite
(P)*
|
Li2O.Al2O3.8SiO2
|
4.88
|
16.65
|
78.47
|
612.48
g
|
|
|
|
Other Minerals
:
|
Chemical
Formula
|
Empirical
Formula
|
Li%
|
Molar
Mass
|
|
Amblygonite
|
(Li,Na)Al(PO4)(F,OH)
|
Li0.75
Na0.25 Al(PO4)
F0.75
(OH)0.25
|
3.44
|
147.90
g
|
|
Lepidolite
|
K(Li,Al)3(Si,Al)4
O10
(F,OH)2
|
KLi2AlSi4O10F(OH)
|
3.58
|
388,30
g
|
|
-
|
|
Chemical
Compounds :
|
-Chemical
Formula
|
Li2O%
|
CO2%
|
Li%
|
Molar
Mass
|
|
Lithium
carbonate
|
Li2CO3
|
40.44
|
59.56
|
18.74
|
73.89
g
|
|
Lithium
fluoride
|
LiF**
|
-
|
-
|
26.75
|
25.94
g
|
-
- * : See ternary diagram
hereafter
- ** : 1 mole of LiF (25.94
g) will give 0.5 mole of Li2O in the ceramic product
that is to say 14.94 g
-
-
-
- Ternary diagram
Li2O.Al2O3.SiO2 (L.A.S) :
-
- The ternary diagram
Li2O-Al2O3-SiO2 provides important information
concerning the useful phases for the design of
vitroceramics.
-
- Vitroceramics are ceramic
materials obtained by a controled process of
nucleation-growth applied to an " Ad hoc " vitreous
matrix. These vitroceramic materials are those which
have been most largely used because of their low
dilatation coefficients, null or negative and also for
their thermal and chemical stability.
-
- Examples :
- - Lithium enters culinary
vitroceramics for cooking which go on the flame (ex:
famous pans of amber glass…).
- - Lithium is also used for
the optical vitroceramics intended for telescopes of
utmost precision because they require an extremely low
linear dilatation coefficient.
-
-
 - Phase diagrams
Li2O - Al2O3 - SiO2
- with indication
of the vitreous zones in yellow,
- of the lithium
compounds P = Petalite, R = Lithium Feldspar, S =
Spodumene, E = Eucryptite
- and of the zones
with negative thermal dilatations I and II in green
-
-
-
-
- The vitreous zone in this
ternary system is crossed by that of minerals with low
or negative thermal dilatation coefficients (points P,
R, S, E in the zones in green). The vitreous compounds
located along the Li2O-Al2O3-SiO2 line present an
aptitude to produce fast crystallizations in -
eucryptite and in - spodumene.
-
-
-
- Extracts of summaries of
publications by V.G. AVETIKOV in connection with
porcelain bodies containing spodumene
:
-
-
- " The ternary system
Li2O-Al2O3-SiO2 (L.A.S.) contains many compounds like
eucryptite (Li2O-Al2O3-2SiO2), spodumene
(Li2O-Al2O3-4SiO2), lithium feldspar
(Li2O-Al2O3-6SiO2) and petalite (Li2O-Al2O3-8SiO2).
These various mineral compounds of lithium are usable
in ceramic pieces."
-
- " Several of these
compounds, and in particular spodumene, used in the
development of ceramic bodies by addition of
argillaceous and mineral matters give very weak
thermal dilatations, and even negative in the range of
temperature going from 0 to 800°C."
-
- => This is what one seeks
with the special bodies that go on the flame.
-
- " For example, when one
replaces pegmatite or feldspar of a porcelain body by
spodumene, the coefficient of dilatation may be
lowered by 2 to 3 times compared to its value in a
feldspathic product."
-
- " In porcelain bodies in
which Li2O was added up to a total value of 2%, the
mechanical properties and the electrical resistance of
the shapes containing spodumene approach those of the
porcelains for high voltage (high voltage insulators).
The thermal dilatation coefficient is lower (1.5 to
2.5 x10-6 °C-1) in the range of 0 to
200°C."
-
- " A special attention is
to be paid to the conditions of cooling of the
products after firing. The pieces containing
approximately 2% Li2O which cool too slowly have
altered structures and are not very dense, their
dilatation is negative. V.G. AVETIKOV concludes that
this is due to recrystallizations of lithium
aluminosilicates caused by a too slow cooling."
-
- " To obtain dense
porcelain structures, the speed of cooling between
1300°C and 1100°C must be approximately
300°C per hour. Under these conditions the
thermal dilatation coefficient remains positive. The
speed of cooling under 1100°C does not affect the
quality of the shard."
-
- " The tendency to
recrystallize is directly related to the content in
Li2O."
-
- " The resistance to
thermal shock of the dense shards (not very porous)
increases with the amount of Li2O."
-
-
-
- Lithium favors
nucleation and growth in crystalline glazes :
-
-
- Lithium is generally
introduced in small quantities from 1 to 2% of Li2O in
this type of glaze.
-
- Upon cooling, lithium favors
the formation of crystalline aluminosilicates and in
particular the formation of spodumene from the
ingredients of the vitreous flow. These nucleations
allow to sow the glaze more quickly and improve its
crystallizing capacity.
- These glazes require a very
good control of the cycle of firing cooking in order
to pass to the phase of growth with precision. The
reproducibility of this type of glaze can be rather
difficult.
-
- The crystalline growth is
influenced by the active role of lithium which lowers
the viscosity and the surface tension of crystalline
glazes (*).
-
- A more fluid glaze favors a
faster growth of the crystals. It allows a more
important mobility of the crystallizing elements and
colourants and favors their migration.
-
- The reduction of surface
tension of the glaze favors crystals with low density
structures because these develop themselves and move
more freely on the surface of the glaze. They are to
some extent " more relaxed"…
-
- In short, with lithium low
viscosity more quickly nourishes the crystals and a
lower surface tension favors their not very dense
spreading out on the surface of the glaze. With a time
of growth adapted, a crystalline glaze with lithium (a
few % of Li2O) will rather easily give very large
crystals with diaphanous textures. ("transparency" and
"wings of dragonfly" effects ).
-
-
- It is the same for the other
types of glazes whose viscosity and surface tension
are close to those with lithium.
-
- But attention to glaze
runs…
-
-
 - example of
semi-translucent zinc silicate crystals on a glaze
with lithium (2.5% Li2CO3)
-
-
- (*) : Caution!! Here it
is question of crystalline glazes, therefore of glazes
with heterogeneous phases (vitreous and crystalline).
Calculations of physical properties applied to pure
glasses, such as those of the thermal dilatation
coefficient, superficial tension, elasticity, cannot
be applied to these compositions.
-
-
- Toxicity of lithium :
-
-
- See Edouard Bastarache's
article on lithium (Québec) : Lithium.htm
-
-
- Examples of lithium
containing glazes :
-
-
-
-
CONE 04
Lithium Containing Glazes -
(1060-1080°C) - From : Edouard
Bastarache (https://www.blogger.com/profile/10622613356744391469)
-
- 01
- Sorel Jade Green Glaze
Recipe
by Edouard
Bastarache https://04glazes.blogspot.com/
|

|
- Firing:
Orton cone 04
- North
American raw materials were
substituted for by European
ones.
|
|
Washed
hardwood ash (Oak)
|
36
|
|
Mixed
Feldspar ICE10
|
14.5
|
|
Ferro
Frit 3134 (available in
Europe)
|
23
|
|
Kaolin
|
14.5
|
|
Lithium
carbonate
|
12
|
|
Bentonite
|
2
|
|
Zircon
(Zirconium silicate)
|
15.5
|
|
Black
copper oxide
|
3.5
|
|
Shiny
glaze with areas of surface
microcrystals (spodumene
crystallizations at the time of
cooling).
|
-
- 02
- Ginette's Blue Glaze (My
wife)
Recipe
by Edouard
Bastarache https://04glazes.blogspot.com/
|

|
- Firing
: Orton cone 04
- North
american raw materials were
substitued for european
ones.
|
|
Washed
hardwood ash (oak)
|
35
|
|
Mixed
feldspar ICE10
|
14
|
|
Ferro
frit 3134 (available in
Europe)
|
25
|
|
Kaolin
|
14
|
|
Lithium
carbonate
|
12
|
|
Bentonite
|
2
|
|
Zircon
(Zirconium silicate)
|
15
|
|
Black
cobalt oxide
|
5
|
- Shiny
glaze with areas of surface
microcrystals (spodumene
crystallizations at the time
of cooling).
-
- Richard
Zakin says that we should not
put more than 5% Lithium
Carbonate in a glaze (in his
software Describ9) because
once applied, the dry glaze
can crack and flake
off.
-
- We
checked that and it is true,
then the trick is to rub the
glaze while the shard is still
wet considering the recent
application of the glaze; and
it works.
- As
for Spodumene, it is the same
thing (to flake off). He also
says not to put more than 35%
Spodumene.
|
-
-
- LITHIUM
CRYSTALLINE Glaze for Cone 6
(1220-1240°C) - From John Sankey's
database
(https://www.johnsankey.ca/glazedata.html)
-
- 03 -
Turquoise Crystalline Glaze
Recipe
by Alisa
Clausen https://www.flickr.com/photos/glazes/
|

|
- Firing
: cône cone 6
- Cooling
: fast down to 1100°C then
lowering to 800°C at
80°C per hour
|
|
Potassium
feldspar
|
36
|
|
Zinc
oxide
|
24
|
|
Silica
|
15
|
|
Calcium
carbonate
|
13
|
|
Lithium
carbonate
|
7
|
|
Kaolin
|
5
|
|
Rutile
|
5
|
|
Copper
carbonate
|
1
|
|
Shiny
very fluid glaze with large green
and blue crystals.Looks like an
aerial view of the Caribbean
islands. The crystals have clear
structures like gems.
|
- 04 -
Orange - Blue Crystalline
Glaze
Recipe
by Alisa
Clause https://www.flickr.com/photos/glazes/
|

|
- Firing
: Orton cone 6
- Cooling
: fast down to 1100°C then
down to 800°C at 80°C
per hour.
|
|
Potassium
feldspar
|
36
|
|
Zinc
oxide
|
24
|
|
Silica
|
15
|
|
Calcium
carbonate
|
13
|
|
Lithium
carbonate
|
7
|
|
Kaolin
|
5
|
|
Black
nickel oxide
|
1
|
|
Orange
to rusty glqze background with large
luminous blue crystals and dark blue
centers. The crystals have clear
structures like gems.
|
- GLAZES
containing lithium for cone 9.5
(1280-1300°C) - From : Edouard
Bastarache (https://www.blogger.com/profile/10622613356744391469)
-
- 05 -
White-Rusty Shino
glaze
Recipe
by Edouard
Bastarache
|

|
- Firing
: Orton cone 9.5 in
reduction
|
|
Nepheline
syenite
|
86
|
|
Kaolin
|
3
|
|
Silica
|
4
|
|
Alumina
hydrate
|
3
|
|
Lithium
carbonate
|
4
|
|
Bentonite
|
3
|
|
White
and rusty glaze due to the clay
containing iron.
Lithium
carbonate is added to thwart the
surface flaws generated by the
excess of Nephelite/Syenite (Na2O).
|
- 06 -
Brick Red Shino
Recipe
by Edouard
Bastarache
|

|
|
Firing :
Orton cone 9.5 in
reduction
The North
American raw materials were
substituted for by European
ones.
|
|
Nepheline
syenite
|
42
|
|
Mixed
Feldspar ICE10
|
44
|
|
Kaolin
|
11
|
|
Lithium
carbonate
|
3
|
|
Bentonite
|
4
|
|
Red iron
Oxide
|
2
|
- Textured
brick red glaze
|
|
-
-
-
-
-
-
-
- Smart2000.fr
©
Septembre 2011
-
FRANCE
- Écrit
et documenté par le propriétaire du site
// Contact : Smart2000@wanadoo.fr
- Document
pour CONSULTATION PRIVÉE uniquement - Toute
reproduction totale ou partielle est
interdite
-
-
-
-
- Smart2000.fr
le site
dédié aux passionnés de
céramique
-
- Smart2000
- FRANCE sur https://smart2000.fr/
-
- This
entire page Copyright © 2011-2023, All Rights
Reserved.
- Les
textes et les photos restent la
propriété de leur auteurs, ils ne
peuvent être réutilisés sans un
accord préalable. Nous
consulter.
|









