- Summary------------------------Access
to lanthanide files
-
- On this page :
- Lanthanide colouring oxides in zinc crystalline
glazes
- Introduction
-
- Expérimentation
with oxides of neodymium and praseodymium
-
- Conclusion
-
 French
version French
version-
-
|
LANTHANIDE
COLOURING OXIDES in ZINC CRYSTALLINE
GLAZES
by
Smart2000
|
-
- Translated into english
by Edouard Bastarache
- Sorel-Tracy
- Quebec
- edouardb@colba.net
-
-
-
- INTRODUCTION
:
-
- Lanthanide oxides used for
colouring glass and certain glazes can also be used to
colour zinc crystalline glazes (those which develop
macro-crystals particularly well). These oxides are
mainly those of praseodymium, neodymium, erbium and
cerium.
- Different from transparent
compositions generally used, zinc crystalline glazes
are strongly alkaline and very rich in zinc (0.550 to
0.610 mole).
- The colouring oxides usually
used in zinc macro-crystalline glazes are the basic
oxides such as cobalt, nickel, chromium, iron,
manganese and copper alone or combined in binary,
ternary and sometimes quaternary mixtures. Their
introduction is generally done in small amounts at
rates ranging between 0.05 and 2 %. Under these
conditions the melting effect of these "traditional"
oxides has little incidence on the behaviour of these
glazes whose viscosity could have been deeply affected
by larger amounts. The dissolution of oxides is
highest under these conditions, it allows very
transparent colourings.
- The colouring oxides of
lanthanides are not very powerful in alkaline
compositions high in zinc, their weak colouring
capacity must thus be compensated either by their
introduction in greater amounts or also by more
significant thickness. The limit of solubility of
these oxides (around 7 to 8%) and their rather
powerful melting effect, about that of alkaline
oxides, require a good compromise between the
composition of the glaze base and the maximun of
realizable colouring (thickness applied and quantity
of oxide used) to obtain a suitable crystalline glaze.
-
-
- EXPERIMENTATION
with oxides of praseodymium and neodymium :
-
- 1)
First level of colouring :
-
- In
the examples hereafter, a glaze was coloured with
either black praseodymium oxide 5,5% (Pr6O11) or
neodymium oxide 4,5% (Nd2O3). With equal thickness,
neodymium colours more than praseodymium. The base of
the glaze was not modified compared to its use with
traditional oxides. The fluidity of this glaze (fired
at cone 8/1260°C) was not affected out of the
usual limits. The flow of the glaze in the "catchers"
remained identical to that of the traditional
compositions of crystalline glazes.
-
-
- Example of
a crystalline glaze coloured by
praseodymium (on the left) and neodymium
(on the right).
-
-
- Black
praseodymium oxide (Pr6O11) was introduced
at the 5,5% level into the glaze base and
the lime green colour characteristic of
this colouring material is really not very
constant, except close to the foot of the
piece where the thickness is more
significant.
-
- Neodymium
oxide (Nd2O3) was introduced at the level
of 4,5 % into the glaze base and its
"violine" colour shows better. This
colouring varies (dichroic effect)
according to the source of lighting to
which the piece is exposed. It appears
more blue in daylight and more purplish in
artificial lighting
|

|
|
|

- Thick
transparent coloured glaze deposited on
the bottom of a small cup. Coloured by
black praseodymium oxide 5,5% (Pr6O11
)
|
- The transparent
colouring of the background indicates good
dissolution of the oxides used in these
proportions. The crystals are silvery
white (zinc silicate) and float on the
surface of the coloured glaze. The clear
separation of the molten and crystalline
phases gives rise to products
extraordinarily surprising with changing
tones according to the orientation and the
nature of the light.
-
|
Crystalline
glaze base used
:
|
|
|
|
|
|
CaO
0.079
|
Al2O3
0.083
|
SiO2
1.812
|
|
ZnO
0.598
|
|
B2O3
0.037
|
|
Na2O
0.241
|
|
|
|
K2O
0.022
|
|
|
|
Li2O
0.059
|
|
|
|
-
- 2)
Second level of colouring :
-
- In
the examples hereafter, the glaze was coloured by
black praseodymium oxide 8% (Pr6O11) or with neodymium
oxide 7,5% (Nd2O3). The alkaline base of the glaze was
slightly modified to anticipate the more melting
effect of the oxides used in greater amounts. The
fluidity of this glaze (fired at cone 8/1260°C)
was not affected outside the usual limits. The flow of
glazes in the "catchers" remained identical to that of
the preceding compositions
- The
colour obtained is not more intense, the coloured
background is not transparent any more but opacified
in yellow or mauve in pastel tones, proof that all of
the colouring was not dissolved and that coloured
particles remain in suspension in the molten glaze.
The glaze is saturated by colouring oxides.
-
-
|
|
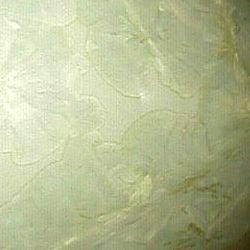
Colour obtained
by black praseodymium oxide 8% (Pr6O11). The
background is not transparent any more, but
opaque and light yellow The colouring
material is not entirely dissolved in the
glaze.
|

Colour
obtained by 7.5% of neodymium oxide (Nd2O3).
The background is not transparent any more,
but opaque and light mauve. The colouring
material is not entirely dissolved in the
glaze.
|
-
- Conclusion
:
-
- This experiment is
enthralling, these oxides little used in ceramics
unceasingly offer new possibilities of colouring for
the unconditional potters fond of macro-crystals in
search of new effects
- The experimentation with
lanthanide oxides in other crystalline glaze bases,
and with higher temperatures, should allow the
discovery of other very spectacular effects with
rather "unreal" colours compared with those obtained
by the traditional range of colouring oxides most
currently used in ceramics.
- The main thing before
launching out in this enthralling work will be mainly
to find a retailer ready to provide you with these
rare lanthanide oxides at reasonable prices.
- If you wish to launch out in
this research and you do not manage to find these
oxides, I will be thankfull that you let me know about
it by sending a message at the following address :
smart2000@wanadoo.fr
-
-
-
-
- Smart2000.fr
©
Février
2003
-
FRANCE
- Écrit
et documenté par le propriétaire du site
// Contact : Smart2000@wanadoo.fr
- Document
pour CONSULTATION PRIVÉE uniquement - Toute
reproduction totale ou partielle est
interdite
-
-
-
-
- Smart2000.fr
le site
dédié aux passionnés de
céramique
-
- Smart2000
- FRANCE sur https://smart2000.fr/
-
- This
entire page Copyright © 2003-2023, All Rights
Reserved.
- Les
textes et les photos restent la
propriété de leur auteurs, ils ne
peuvent être réutilisés sans un
accord préalable. Nous
consulter.
|